
 Antoine Lavoisier
Antoine Lavoisier  Jacques-Louis David’s painting of Antoine and Marie Anne Lavoisier
Jacques-Louis David’s painting of Antoine and Marie Anne Lavoisier
 Priestley’s soda water was briefly touted as a potential cure for scurvy, a disease that plagued sailors on long sea voyages.
Priestley’s soda water was briefly touted as a potential cure for scurvy, a disease that plagued sailors on long sea voyages.
A Tax-Collecting Scientist
Born into a well-to-do Parisian family in 1743, Antoine Laurent Lavoisier received a fine education and, like his father, earned a degree in law. But from an early age he showed more interest in science. At 22, he won a royal prize for an essay laying out a plan for lighting city streets with olive oil, and he did promising early work on geology. His ambition was to become a member of the Royal Academy of Sciences, but the modest salary paid to Academy members would not support the lifestyle to which he was accustomed. So in 1768, with funds inherited upon the death of his mother, he bought a share in the Ferme générale, or General Farm, a private consortium that collected taxes for the king.
As a result of his work as a tax administrator, Lavoisier became a very wealthy man – a multi-millionaire by today’s standards. But his true passion was chemistry. He spent three hours in his private laboratory before work each day, and returned there after dinner … often accompanied by his young wife. Marie Anne Paulze was the daughter of one of Lavoisier's business partners. Under pressure to marry her off to an odious older man, her father had proposed that Lavoisier marry her instead. Though she was only 13 at the time, she brought her own extraordinary talents to the partnership. Among other things, she was a talented artist who created the drawings that accompanied her husband’s published work. And because she spoke English, she served as Antoine’s translator in his dealings with British scientists whose work would inspire his own.
A New Interest in Gases, Inspired by a Spy
While British scientists had made great strides in the study of gases, or what they called “airs,” French scientists had shown little interest in this new area of chemical research. That changed in 1772, when Lavoisier received a tip from a French industrial spy. Britain’s leading expert on gases, Joseph Priestley, had invented a method for making soda water – by injecting “fixed air” (what we now call carbon dioxide) into water. Now the British navy was planning to test the drink as a treatment for scurvy, a disease that plagued sailors on long sea voyages. Quick to pick up on this development was a defrocked Portuguese monk who was camped out in London, monitoring British science on behalf of the French. Sensing a potential military secret, the spy alerted his handler, French Commerce Minister Jean Charles Trudaine de Montigny. Trudaine, in turn, urged Lavoisier to look into the matter without delay. Though soda water would turn out to be useless against scurvy, this tip would awaken Lavoisier’s interest in gases and set him on the path toward his greatest discoveries. He quickly sensed the revolutionary importance of this new field and the threat it posed to chemistry’s prevailing theory.
The Phlogiston Theory
That theory was based on the idea that fire was due to a fiery principle called “phlogiston,” which was given up during combustion. The theory had been the foundation of chemistry for nearly a century, because it seemed to explain not only fire but metals and rust. Metals like iron were formed, the theory said, when their ores were heated in the presence of charcoal, a rich source of phlogiston. When iron rusted, it released its phlogiston, turning into what was then called a “calx.” Other metals went through the same process of “calcination.”
But there was a problem with the theory: Common sense suggested that when iron rusts it must lose weight; after all, the metal becomes brittle and falls apart. But iron actually gains weight when it rusts, and so do other metals when they form calxes – just the opposite of what the phlogiston theory said should happen. How could metals give up their phlogiston and yet get heavier in the process? Other chemists had ignored this anomaly, but Lavoisier, who had an accountant’s obsession with the weights of his experimental ingredients, could not let it pass.
Sound Bite: Tax Farming - Historian Seymour Mauskopf explains “tax farming,” the ancient French practice that made Lavoisier a wealthy man.
Video: Lavoisier’s Better Half - Once a week, Antoine Lavoisier welcomed others into his lab to take part in chemistry experiments, but his most important collaborator was his young wife, Marie Anne.
 French Commerce Minister Jean Charles Trudaine de Montigny was the handler for a spy whose tip about Priestley’s invention of soda water helped spark Lavoisier’s interest in gases.
French Commerce Minister Jean Charles Trudaine de Montigny was the handler for a spy whose tip about Priestley’s invention of soda water helped spark Lavoisier’s interest in gases.
Film Clip: A Revolution In the Making - Phlogiston was the foundation of chemistry’s leading theory for nearly a century ... but there was a flaw in the theory that prompted Lavoisier to investigate.
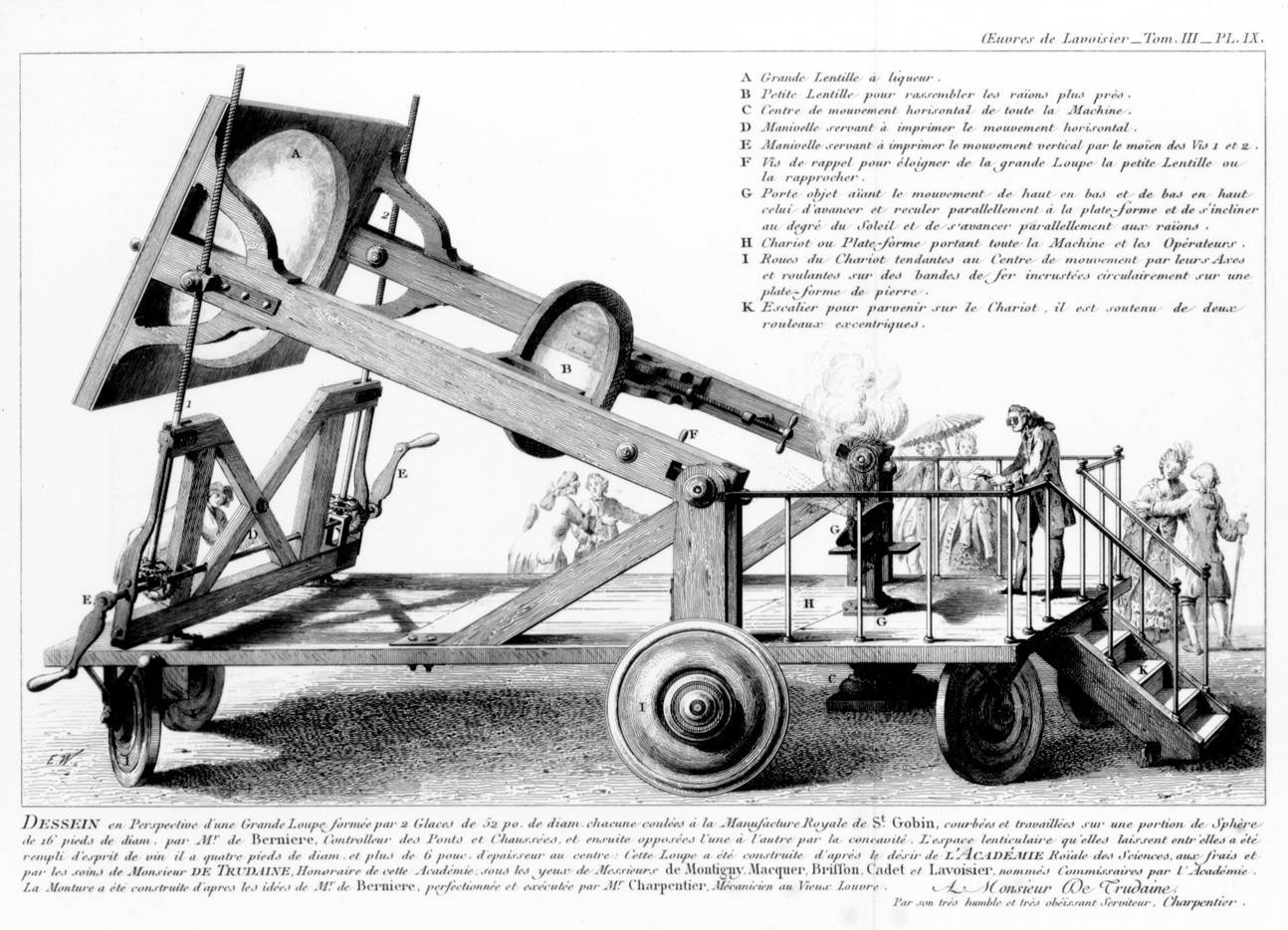 Lavoisier’s Burning Lens (Click to Enlarge)
Lavoisier’s Burning Lens (Click to Enlarge)
 In 1774, Priestley discovered a new gas that made candles burn bigger and brighter than ordinary air.
In 1774, Priestley discovered a new gas that made candles burn bigger and brighter than ordinary air.
Solving the Riddle of Rust
In October 1772, a few months after receiving the tip about Priestley’s soda water, Lavoisier set out to explain the longstanding puzzle of why metals gain weight when they form calxes. Lavoisier conducted a series of public experiments, relying on a huge burning lens that focused the sun's rays to produce intense heat … while elegantly dressed bystanders watched in amazement. Lavoisier found that a calx of lead, mixed with charcoal and heated by the sun’s rays, gave off a large amount of air as it turned back into metallic lead. This suggested that air, or some part of the air, might somehow responsible for calxes’ being heavier than expected. Lavoisier also found that when he burned elements like sulfur, they, too, gained weight, apparently by absorbing the same gas. For two years, Lavoisier labored in vain to identify this mystery gas. Then, in October 1774, the answer would be delivered by none other than Joseph Priestley.
News of a Mysterious New Air
In England, Priestley had begun to study a curious substance called the red calx of mercury. When heated, this red powder turned back into liquid mercury and gave off a gas. Priestley collected the gas, expecting it to be “fixed air,” which would put out a candle. To his surprise, this gas did just the opposite. It made candles burn bigger and brighter than ordinary air. Priestley was at a loss to explain this finding – and eager to share it with all who would listen. On a trip to Paris two months later, Priestley was invited to dine with the members of the Royal Academy of Sciences. Lavoisier listened with great interest as Priestley described the curious new air he’d discovered. Could this be the gas he’d been looking for – the one involved in rusting and burning? After Priestley left, Lavoisier hurried to the local apothecary to buy his own sample of mercury calx and begin his own experiments on it.
Film Clip: Some Kind of Air - Lavoisier discovered that air, or some part of the air, is responsible for calxes’ being heavier than expected.
Film Clip: Dinner with Priestley - Over dinner in October 1774, Priestley told Lavoisier about the new air he’d discovered.
 With the help of a mouse, Priestley discovers this air is more “light and easy” to breathe than ordinary air.
With the help of a mouse, Priestley discovers this air is more “light and easy” to breathe than ordinary air.
Better than Air
Back in England, Priestley continued to explore his new gas. Since it supported fire, he wondered, might it also support breathing? To find out, he trapped a mouse under a glass vessel containing the new air. To his amazement, the mouse seemed perfectly at ease long after it would have died in normal air. This air was somehow better than normal air. Emboldened by the mouse’s experience, Priestley tried the gas himself, noting that he felt “peculiarly light and easy” after inhaling it. Up to that moment, Priestley declared, “only two mice and I have had the privilege of breathing it.”
What Priestley didn’t realize is that he now had a competitor on his heels. In Paris, acting on Priestley’s revelations over dinner, Lavoisier was performing many of the same experiments, and in April 1775, he announced to the Royal Academy that he had discovered a new air “more pure than even the common air in which we live.” He would soon give it the name “oxygen.”
Film Clip: Oxygen - Tipped off by Priestley, Lavoisier began his own experiments and soon announced his discovery of a “pure” air he called oxygen.
Air, Water, Earth and Fire Redefined
Lavoisier was not the first to isolate oxygen. Priestley had beaten him to it by about six months, and another chemist named Carl Wilhelm Scheele, working as an apothecary in Sweden, had described the same gas (he called it “fire air”) even earlier, in 1771. But both Priestley and Scheele interpreted their findings within the context of the prevailing phlogiston theory. Only Lavoisier recognized that this new gas meant the end of the old theory.
Over the next 15 years, he would show that oxygen was a key ingredient in both air and water ... that fire was a process of chemically combining with oxygen ... and that oxygen was bound up in every imaginable sort of rock. There was no telling how many new elements chemists might discover if they could release it from ores. Since phlogiston wasn’t needed to explain any of this, Lavoisier argued it was an imaginary substance deserving of the dust bin.
Film Clip: Lavoisier’s Discoveries - Lavoisier made oxygen the foundation of a whole new chemistry and showed there was no need for phlogiston.
 Lavoisier’s revolutionary textbook
Lavoisier’s revolutionary textbook
The Chemical Revolution
For years, many chemists – including Joseph Priestley – refused to abandon the phlogiston theory. What finally turned the tide was the textbook Lavoisier wrote in 1789 to spread his oxygen-inspired views. The book included a whole new chemical language, throwing out the fanciful, antiquated terms of alchemy. In their place Lavoisier and his disciples proposed a spare, utilitarian nomenclature in which the name of each compound reveals its constituent elements. (Today’s chemical language also reveals their ratios: Carbon dioxide = one atom of carbon bonded with two atoms of oxygen.) As the book and the language were adopted around the world, phlogiston quietly passed into history. This marked the end of the Chemical Revolution – and the beginning of a worldwide race to discover new elements.
How Lavoisier Helped America Win the Revolutionary War
Lavoisier was a talented administrator, as well as a gifted scientist, so in 1775, when the French government set out to reform its munitions bureau, Lavoisier was given an apartment in the Paris Arsenal and tapped to lead the effort. Charged with improving the production of French gun powder, he would play a key role in the American Revolution. More than 800 tons of Lavoisier’s finest French powder were shipped to America, helping to turn the tide in the colonists’ struggle to win their independence from Britain.
 Like King Louis XVI before him, Lavoisier was executed by guillotine along with his father-in-law and 26 other “tax farmers” after the French Revolution.
Like King Louis XVI before him, Lavoisier was executed by guillotine along with his father-in-law and 26 other “tax farmers” after the French Revolution.
Taxes and Death
Lavoisier brought the same zeal to his work as a tax administrator that he did to his chemistry, and in time this would make him one of the least popular people in Paris. Realizing that the smuggling of goods into Paris was costing the crown about 20 percent of its tax revenue, Lavoisier proposed that a wall be built around the city, with entry gates where traders could be stopped, searched and forced to pay a tax. The hated wall, which led to higher taxes for ordinary Parisians, was built in 1787, so it was still fresh in the minds of the revolutionaries who stormed the Bastille two years later.
Though Lavoisier survived the rebels’ initial attack on the Arsenal, he and 27 of his fellow tax farmers – strongly linked with the old regime – were eventually tried and convicted. On May 8, 1794, immediately after Marie Anne’s father, Lavoisier was executed by guillotine, prompting one fellow scientist to remark: "It took them only an instant to cut off that head, and one hundred years might not be sufficient to produce another like it.”
Video: Antoine Lavoisier, American Ally - Lavoisier’s French gunpowder helped American colonists turn the tide in the Revolutionary War.
 Chemists Roald Hoffmann and Carl Djerassi and their play Oxygen
Chemists Roald Hoffmann and Carl Djerassi and their play Oxygen
 By paying close attention to the weights of his experimental ingredients, Lavoisier made the Conservation of Matter a fundamental principle of chemistry.
By paying close attention to the weights of his experimental ingredients, Lavoisier made the Conservation of Matter a fundamental principle of chemistry.
Who Discovered Oxygen?
Centuries later, scholars continue to debate who deserves credit for discovering oxygen. Should it be Priestley, who brought the world’s attention to the new gas? Or Lavoisier, who understood what the new gas meant? Or Scheele, who was the first to discover the gas but didn’t publish his results until after Priestley and Lavoisier? This question about the meaning of “scientific discovery” is explored in the play Oxygen, by chemists Carl Djerassi and Roald Hoffmann.
The Conservation of Matter
On one thing there is no debate: It was Lavoisier, and no one else, who firmly established the principle we now call the Conservation of Matter – the idea that matter is neither created nor destroyed during a chemical process. Lavoisier’s obsessive attention to the weights of his experimental ingredients allowed him to make many of the discoveries for which he’s remembered today. And more than two centuries after his death, this principle remains the bedrock of chemistry.
Want to Learn More?
To see the full oxygen story, select Watch Online to go to pbs.org/mysteryofmatter and view Episode 1. Or click on Buy to purchase the series DVD at ShopPBS. For more resources about Antoine Lavoisier’s life and career, select Learn More.
Video: Who Discovered Oxygen? - The story of oxygen raises fascinating questions about the meaning of scientific discovery. Video: The Conservation of Matter - As he struggled to amass evidence for a discovery that threatened to topple chemistry’s reigning theory, Antoine Lavoisier invented a powerful laboratory technique based on the principle now called the Conservation of Matter: The same amount of matter exists before and after each chemical reaction – what comes out has to weigh exactly as much as what goes in. More than two centuries later, this principle remains a bedrock of chemistry.