
 Joseph Priestley
Joseph Priestley
Birth of a Scientist
Joseph Priestley was a man of wide interests and boundless curiosity. The son of a lower middle-class cloth finisher, he was born in 1733 in Yorkshire, England, about 200 miles north of London. Raised outside England’s state religion, he was educated at one of the “dissenting academies” known for their academic rigor and free thinking, and would eventually become one of the founders of the Unitarian Church. While holding a variety of jobs as a minister, teacher, lecturer and tutor, he wrote prolifically on subjects ranging from education and theology to politics and science. One of his early books – The History and Present State of Electricity – led to Priestley’s warm friendship with Benjamin Franklin and inspired him to become a scientist in his own right.
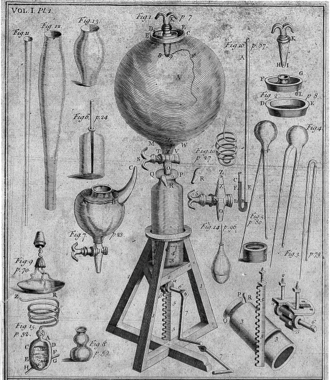 Boyle's Air Pump
Boyle's Air Pump
The Discovery of Air
From electricity Priestley next turned his attention to the study of air. For centuries, scientists (then called natural philosophers) had paid little attention to air. It seemed to be just the empty space between the things that interested them. But when the invention of the air pump in the mid-1600s allowed scientists like Robert Boyle to create artificial vacuums, they realized air was more than empty space. This led to new discoveries about air – and new devices for trapping and testing it. The big breakthrough came in 1754, when a Scottish medical student named Joseph Black discovered “fixed air” – the gas we now call carbon dioxide. Its properties were so remarkable that scientists realized for the first time that there was more than one kind of air – that gases represented a whole new dimension of matter to be explored. British scientists leapt into the study of “airs,” quickly discovering new gases like hydrogen and nitrogen. But they were only setting the stage for Priestley’s historic discoveries in this field.
Video: The Discovery of Air - How air came to be a subject scientists thought was worth studying.
 Priestley’s experiments at a nearby brewery led to this invention of soda water.
Priestley’s experiments at a nearby brewery led to this invention of soda water.
Soda Water
In 1767, Priestley left his position as a tutor in Warrington to take up a new post as a minister farther north in Leeds. The move would have historic repercussions for the history of science. When the home he had purchased was not quite ready on his arrival, Priestley’s new congregation put him up in a house near a brewery. Intrigued by this convenient source of carbon dioxide, Priestley began a series of experiments that led to his invention of a method for making soda water – carbonation. While others (like Joseph Schweppe) took steps to capitalize on his discovery, making fortunes in the process, Priestley blithely shared his discovery with the world. The mistaken idea that his “windy water” might be a cure for scurvy was picked up by a French industrial spy who was scouting out the England’s Royal Society for potential military secrets. He passed it on to France’s brightest young chemist, Antoine Lavoisier. Though soda water would turn out to be useless against scurvy, this tip would set Lavoisier on the path toward his great discoveries – and put him on a collision course with Priestley.
Film Clip: Windy Water - How Priestley’s invention of soda water triggered a chain of events that changed the course of science.
 Antoine Lavoisier
Antoine Lavoisier
The Phlogiston Theory
Lavoisier sensed immediately that the new science of gases had the potential to bring about a scientific revolution that would topple chemistry’s reigning theory. That theory was based on the idea that fire was due to a fiery principle called “phlogiston,” which was given up during combustion. Today the phlogiston theory is seen as a classic example of a misguided scientific idea, but it was a very plausible and successful theory in its day, because it seemed to explain not only fire but also metals and rust. Metals were formed, the theory said, when their ores were heated in the presence of charcoal, a rich source of phlogiston. When those metals rusted, they released their phlogiston, turning into what was then called a “calx.” Chemists could turn the calx back into a metal by heating it with charcoal.
Film Clip: Phlogiston Theory - Phlogiston was the foundation of chemistry’s leading theory for nearly a century ... but there was a flaw in the theory that prompted Lavoisier to investigate.
 The red calx of mercury
The red calx of mercury
A Mysterious New Gas
By 1774, Priestley had begun to experiment with a curious substance called the red calx of mercury. This red powder seemed to contradict the phlogiston theory, because chemists could turn it back into liquid mercury simply by heating it. No charcoal – no source of phlogiston – was needed. This was theoretically impossible. The ever-curious Priestley wanted to know how it could happen. So in August 1774, he obtained a sample of mercury calx and used a burning lens to heat it with sunlight. What he found was a remarkable new gas (he called it “dephlogisticated air”) that made candles burn brighter and longer than ordinary air. Two months later, he shared his findings over dinner with Lavoisier in Paris.
Film Clip: A Mysterious New Gas - Priestley finds a new gas in which candles burn longer and brighter than in ordinary air.
 Priestley and Lavoisier at dinner.
Priestley and Lavoisier at dinner.
A Man and a Mouse
Acting on the tip about Priestley’s method for making soda water, Lavoisier had begun his own study of “airs” and made a discovery that threatened to overturn the phlogiston theory – the very foundation of chemistry. Paying close attention to the weights of his experimental ingredients, Lavoisier noticed that metals gain weight as they rust. Even though theory said phlogiston was leaving the metal, the resulting calx was heavier than the metal from which it formed. Puzzled, Lavoisier began a series of experiments that revealed the source of this weight gain: Air, or some part of air, was absorbed into the metal as it rusted. This same “air” seemed to be absorbed by objects as they burned.
For two years, Lavoisier had searched in vain for the identity of this mystery gas. But as he listened to Priestley describe his remarkable new gas over dinner in October 1774, he wondered if this was the gas he’d been looking for. Lavoisier hurried to the local apothecary to buy his own sample of mercury calx. Meanwhile, Priestley returned to England and, with the help of a small friend, discovered that this new gas made his lungs feel “peculiarly light and easy” when breathed in.
Film Clip: "Super Air" - With the help of a mouse, Priestley discovers this air is more “light and easy” to breathe than ordinary air.
 Priestley rebuking Lavoisier for not mentioning him.
Priestley rebuking Lavoisier for not mentioning him.
 Carl Wilhelm Scheele
Carl Wilhelm Scheele
 Chemists Roald Hoffmann and Carl Djerassi and their play Oxygen
Chemists Roald Hoffmann and Carl Djerassi and their play Oxygen
Lavoisier's Oxygen
Across the English Channel, Lavoisier was performing his own experiments on mercury calx, discovering the same properties Priestley had observed. This new gas, which Lavoisier would soon name “oxygen,” was “more breathable, more combustible and more pure than even the common air in which we live.” He announced his findings with great fanfare at the 1775 Easter meeting of the French Academy of Sciences. But in a move that is still earning him criticism more than two centuries later, Lavoisier failed to acknowledge his debt to Priestley.
Who Discovered Oxygen?
While it was Priestley who isolated this new gas, it was Lavoisier who grasped its profound implications. Over the next 15 years, he would make oxygen the foundation of a whole new chemistry, showing that it was a key ingredient in both air and water, and that fire was not an element, as the ancients believed, but a process of combining with oxygen. Lavoisier’s discoveries would eventually overthrow the phlogiston theory – a classic example of a scientific revolution. Yet Priestley, like many any other chemists of the day, stubbornly clung to the old theory, prompting one contemporary to call him “the father of modern chemistry who never acknowledged his daughter.”
Centuries later, scholars continue to debate who deserves credit for discovering oxygen. Should it be Priestley, who brought the world’s attention to the new gas? Or Lavoisier, who understood what the new gas meant? Or a third man, Carl Wilhelm Scheele, a Swedish apothecary who was actually the first to discover the gas he called “fire air” but who didn’t publish his results until after Priestley and Lavoisier? This question about the meaning of “scientific discovery” is explored in a play called Oxygen by chemists Carl Djerassi and Roald Hoffmann – and in the video Who Discovered Oxygen?
 With the help of a “sprig of mint,” Priestley discovered the role plants play in keeping air breathable.
With the help of a “sprig of mint,” Priestley discovered the role plants play in keeping air breathable.
Keeping the Atmosphere Sweet
While Priestley never accepted the revolutionary importance of his own discovery, he did make a critical finding about the importance of oxygen to life on earth. In a disarmingly simple experiment with what he called a “sprig of mint,” he discovered – with help from his friend Benjamin Franklin – the vital role that plants play in keeping the earth’s atmosphere fit for animal life through the process we now call photosynthesis. It was a fundamental contribution to environmental science.
Intellectual Exile
As his scientific activity waned, Priestley took an ever greater interest in politics, offering vocal support for the American cause in the Revolutionary War and, a decade later, for the French rebels who overthrew King Louis the XVI. By then, Steven Johnson says, Priestley was “one of the most dangerous minds in all of England,” and he would pay a heavy price for his outspoken liberal views. In 1791, on the second anniversary of Bastille Day, a British mob, incensed at Priestley’s support for the French Revolution, burned down his home in Birmingham. He fled to America and spent the last ten years of his life in Pennsylvania. By doing so, Johnson notes, Priestley set a precedent for the many other intellectual exiles who would follow.
Want to Learn More?
To see the full Priestley story, select Watch Online to go to pbs.org/mysteryofmatter and watch Episode 1. Or click on Buy to purchase the series DVD at ShopPBS. For more resources about Joseph Priestley’s life and career, select Learn More.
