
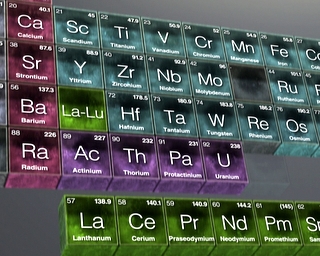
 Uranium was long thought to be the largest of the chemical elements.
Uranium was long thought to be the largest of the chemical elements.
 University of California chemist Glenn Seaborg was the leader of a worldwide effort to create elements beyond uranium.
University of California chemist Glenn Seaborg was the leader of a worldwide effort to create elements beyond uranium.
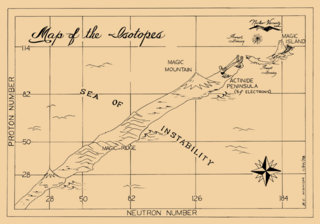 In the late 1960s, Seaborg proposed that out beyond element 110 lay an “Island of Stability” where long-lived superheavy elements still await discovery.
In the late 1960s, Seaborg proposed that out beyond element 110 lay an “Island of Stability” where long-lived superheavy elements still await discovery.
 Maria Goeppert Mayer was one of the scientists who proposed that neutrons and protons are arranged in shells within the nucleus. She shared the 1963 Nobel Prize in physics – only the second woman to win one.
Maria Goeppert Mayer was one of the scientists who proposed that neutrons and protons are arranged in shells within the nucleus. She shared the 1963 Nobel Prize in physics – only the second woman to win one.
 Physicist Eugene Wigner, who shared with 1963 Nobel Prize with Maria Goeppert Mayer, coined the term “magic numbers” to describe the most stable configurations of neutrons and protons.
Physicist Eugene Wigner, who shared with 1963 Nobel Prize with Maria Goeppert Mayer, coined the term “magic numbers” to describe the most stable configurations of neutrons and protons.
 Element 118 (as yet unnamed) is the heaviest known element – so far.
Element 118 (as yet unnamed) is the heaviest known element – so far.
 When a metal called germanium was discovered in 1886, no one had any idea it would one day be a pivotal part of our electronic devices.
When a metal called germanium was discovered in 1886, no one had any idea it would one day be a pivotal part of our electronic devices.
Going Beyond Uranium
For more than a century after its discovery in 1789, uranium (atomic number 92) was thought to be the heaviest of the chemical elements – the last stop on the Periodic Table. But over the last 75 years, more than 25 “transuranic” elements have been added to the table, starting with neptunium (93) in 1940 and, most recently, element 118 in 2005.
The leading figure in these events was a University of California chemist named Glenn Seaborg, who created plutonium (94) in 1941 and two more artificial elements, americium (95) and curium (96), even as he worked on the Manhattan Project during World War II. Back at Berkeley after the war, Seaborg and his team continued their quest, bombarding heavy elements with smaller ones in hopes they would fuse to create a brand new form of matter. In this way, they created five more elements in the next ten years – including berkelium (97) and californium (98). Scientists from the Joint Institute for Nuclear Research in Dubna, Russia, soon joined the hunt, and together these two teams stretched the Periodic Table up to element 106 – named seaborgium in Seaborg’s honor.
But even as these new entries were being added to the table, the element hunters noticed a disturbing trend: The heavier the element they created, the shorter its life span. The few atoms they managed to produce quickly decayed into stabler elements, sometimes in just fractions of a second. If this trend continued, the search for new elements would soon come to an end.
A Magical Isle
But in the late 1960s, Seaborg held out hope that this trend would eventually reverse itself. Out beyond element 110, he predicted, they would find an "Island of Stability," where long-lived elements still await discovery.
Fanciful as it sounded, Seaborg's idea was grounded in a new theory about the atom – that the protons and neutrons at the center of each atom are arranged in shells within the nucleus, much as electrons are arranged in shells around it. “Each shell can hold only so many neutrons or protons,” explains MIT historian David Kaiser. “And when a nucleus has just the right number to fill up its outer shell, the atom is highly stable – just as a full shell of electrons makes for stable, unreactive elements like the noble gases.”
The nuclear shell theory predicted that superheavy elements with the “magic number” of protons or neutrons needed to fill their respective shells would enjoy longer lives. For neutrons, the magic number was 184; for protons, the numbers 114, 120 and 126 looked promising. An element that combined the right number of neutrons and protons would be “doubly magic” and the most stable of all.
Inspired by this idea, the American and Russian element hunters – soon joined by a team from GSI Helmholtz Centre for Heavy Ion Research in Darmstadt, Germany – pushed into new reaches of the Periodic Table. “No effort or expense was spared in this enterprise,” noted the late Oliver Sacks. “The vast atom-smashers of Berkeley, Dubna and Darmstadt were all enlisted in the quest, and scores of brilliant workers devoted their lives to it.”
Sighting the Island
In the 1980s, the Germans surged into the lead, creating six consecutive elements, from 107 (bohrium) to 112 (copernicium). But it wasn’t until 1998 that a team from Dubna and California's Lawrence Livermore National Laboratory finally sighted the fabled island. Firing a beam of neutron-rich calcium (20) ions at a plutonium (94) target, they managed to create a few atoms of an element with the magic number of 114 protons. Though they fell nine neutrons short of the magic number of 184, the half-life of this new element, flerovium, was a whopping 30 seconds. “Thirty seconds may not sound like a lot,” says chemist Ken Moody, a member of the Lawrence Livermore team, “but it showed that the Island of Stability is real – the magic numbers do result in longer-lasting superheavy elements, just as Seaborg predicted.”
How much more stable would element 114 be if team leader Yuri Oganessian and his colleagues could find a way to pack in those nine extra neutrons? No one knows. But theorists predict some isotopes of superheavy elements might last hundreds, even thousands, of years.
Since 1998, the Dubna-Livermore team has caught fleeting glimpses of four more elements (115 through 118), inching closer to the magic number of 184 neutrons and finding further evidence of the Island of Stability's whereabouts. “We're not there yet,” Moody says, “but we're sort of mapping the shoals.”
Why Search?
Some people – even some chemists – question the enormous effort and billions of dollars required to create these fleeting forms of matter. Why go to such trouble and expense to create new elements that are often gone, as Moody puts it, "before you even know you had them"?
Proponents of the research make two arguments in its defense. The first is practical. They point out that long-lived superheavy elements, if they’re ever created, might have uses we can’t even imagine. History is filled with examples of seemingly impractical scientific discoveries that turned out to have important applications. When chemist Clemens Winkler discovered an obscure element called germanium in 1886, for example, he had no inkling it would later be a key ingredient in computer chips. “The quest just to know often has led to amazing spinoffs and practical things that have made all our lives much better,” Kaiser points out.
The second justification of the quest for Seaborg’s legendary island is more philosophical: It’s important because the goal of science is to keep pushing the boundaries of human knowledge, regardless of its practical value. “We search for the Island of Stability because, like Mount Everest, it is there,” Oliver Sacks once wrote. “The quest for the magic island shows us that science is far from being coldness and calculation, as many people imagine, but is shot through with passion, longing and romance.”
 Seaborg’s principal collaborator in the search for new elements was Albert Ghiorso. Recruited to work on the Manhattan Project during World War II, Ghiorso returned to Berkeley with Seaborg and became co-discover of more elements than anyone in history.
Seaborg’s principal collaborator in the search for new elements was Albert Ghiorso. Recruited to work on the Manhattan Project during World War II, Ghiorso returned to Berkeley with Seaborg and became co-discover of more elements than anyone in history.
 Element hunters Glenn Seaborg and Albert Ghiorso from the University of California eventually joined forces with researchers from the Joint Institute for Nuclear Research in Dubna, Russia, including Georgii Flerov and Yuri Oganessian.
Element hunters Glenn Seaborg and Albert Ghiorso from the University of California eventually joined forces with researchers from the Joint Institute for Nuclear Research in Dubna, Russia, including Georgii Flerov and Yuri Oganessian.Sound Bite: Nuclear Shells – Historian David Kaiser explains how discoveries in the 1950s led to the realization that neutrons and protons are arranged in discrete shells within the nucleus, just as electrons are arranged in shells around it.
Sound Bite: Magic Numbers – The nuclear shell theory predicted that certain numbers of neutrons and protons would make even the largest of atoms stable.
Sound Bite: Signs of Stability – A huge, worldwide effort waged since the 1960s has resulted in colossal new elements – and signs that the magic numbers of neutrons and protons do indeed make superheavy elements more stable.
Sound Bite: A Test of Our Knowledge – David Kaiser points out that the effort to create superheavy elements is a test of how well scientists understand the natural laws that govern how matter is put together.
Sound Bite: The Quest to Know – David Kaiser notes that the pursuit of knowledge for its own sake has often resulted in unexpected practical benefits.